UNIT NO.4
Structure of an Atom
(Solved Exercise)
4.2 Write short answers.
1. What is atom?
An atom is the smallest particle of an element that can take part in a chemical reaction.
2. Define element.
An element is a substance that consists of only one kind of atoms. For example Hydrogen, Iron, Gold etc.
3. What type of charge is present on neutron?
There is no charge on neutron.
4. What do you mean by atomic number?
Atomic number (Z)
The number of protons present in an atom of an element is called atomic number of that element. It is denoted as Z. All the atoms of an element have the same number of protons.
5. What is mass number?
Mass number (A)
The total number of protons plus neutrons present in an atom of an element is called mass number of that element. It is denoted as A.
4.3 Constructed Response Questions
1. Oxygen is a non-metallic element.
(a) What is atomic number of oxygen?
Atomic number of oxygen is 8.
(b) In which group of the Periodic Table, oxygen is located?
Oxygen is located in 16th group of the Periodic Table.
(c) In which period of the Periodic Table, oxygen is located?
Oxygen is located in 2nd period of the Periodic Table.
(d) How many electrons are required by oxygen atom to complete its valence shell
Oxygen atom needs 2 electrons to complete its valence shell.
2. Sodium is a metallic element.
(a) What is symbol of sodium?
Symbol of sodium is Na.
(b) What is atomic number of sodium?
Atomic number of sodium is 11.
(c) What is the period number of sodium in Periodic Table?
Period number of sodium is 3 in Periodic Table.
(d) What is the group number of sodium in Periodic Table?
Group number of sodium is 1 in Periodic Table.
(e) Name the family of metals, sodium belongs to.
Sodium belongs to Alkali metals.
3. Name the members of the following families in the Periodic Table:
(a) Alkali metals
Lithium (Li), Sodium (Na), Potassium (K), Rubidium (Rb), Cesium (Cs), Francium (Fr).
(b) Alkaline earth metals
Beryllium (Be), Magnesium (Mg), Calcium (Ca), Strontium (Sr), Barium (Ba), Radium (Ra).
(c) Halogens
Fluorine (F), Chlorine (Cl), Bromine (Br), Iodine (I), Astatine (At).
(d) Nobel gases
Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe), Radon (Rn).
4. Name the element having the same period number and group number.
Hydrogen has same period number and group number.
13
5. What does 27Al indicate?
Aluminum has 13 atomic number and 27 mass number.
6. Why do H and Be exist as atoms while He and Ne as molecules?
Because Hydrogen and Beryllium are different than Helium and Neon according to their electronic configuration. Helium and Neon have complete their valence shell.
7. What does electronic configuration mean?
The distribution of electrons around the nucleus in various shell according to their increasing energy is called electronic configuration.
8. What is the difference between an atom and isotope?
Isotopes are the atoms of an element that have same atomic number but different mass number, while atoms have same atomic number and mass number.
4.4 Investigate the isotopes and their uses.
There are many isotopes of the elements in periodic table like the isotopes of hydrogen, chlorine, carbon, cobalt etc. These isotopes are used in the fields of medicine, radiotherapy, to determine the age of fossils, for the treatment of skin cancer and tumors in human body etc. Some isotopes are also used for the generation of electricity like uranium.
4.5 Project
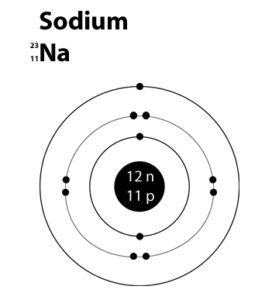
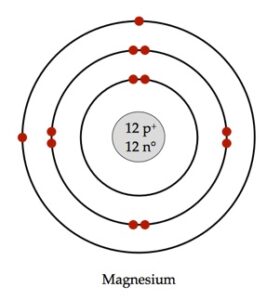
1. Draw the atomic structures of sodium and magnesium having atomic number 11
and 12 respectively.
Atomic Structures of Sodium
Atomic Structures of Magnesium
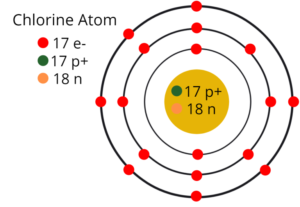
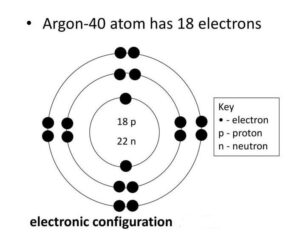
2. Draw the atomic structures of chlorine and argon having atomic number 17 and 18.
Atomic Structures of Chlorine